Using Cipher system to map the lithium content in Li-ion battery cathode materials
Cipher® is used to map the lithium distribution in the scanning electron microscope (SEM). Through a combination of energy dispersive x-ray spectroscopy (EDS) and quantified backscatter electron (qBSE) imaging, the Li content in a metal-oxide cathode material is estimated. The mean Li content was found to be approximately 23.8 at.%.
Introduction
In recent years, Lithium- (Li-) based products have gained significant market acceptance in a diverse range of energy storage applications due to their superior capacity and lighter mass. Today, many different materials and chemistries are employed—or being developed— for anodes and cathodes within an electrochemical cell; lithium iron phosphate and lithium nickel manganese cobalt oxides (NMC) account for approximately two-thirds of the global demand for cathodes in Li-ion batteries. To understand and optimize the properties of cathodes used in battery applications, it is desirable to correlate and control the structural properties and elemental composition of these materials and study their evolution over a battery’s lifetime. However, there are few tools available to measure the inter- or intra-particle lithium content at an appropriate spatial resolution making it difficult to monitor critical processes that result in the degradation of a cell.
Typically, NMC materials used in the construction of battery cathodes are agglomerate oxide particles which, in the uncharged cell state, contain ~ 25 at. % Li. NMCs have a chemical formula Li(NixMnyCoz)O2. They are commonly described by the ratio of nickel to manganese to cobalt, e.g., NMC xyz (where xyz represents the ratio of nickel to manganese to cobalt). However, commonly used microscale elemental analysis techniques, like X-ray energy dispersive spectroscopy (EDS), are unsuitable for Li detection in battery materials as the probability that a characteristic Li X-ray may be generated depends on the bonding state of the Li-ion; many researchers have noted that although specialized EDS detectors can be used to detect X-rays from metallic lithium, no Li X-rays are generated when the lithium is bonded to oxygen—a considerable barrier when considering lithium metal oxides used in cathode materials.
The proliferation of research in Li-based energy storage technologies insists on techniques for reliable microscale detection and quantification of Lithium content. Li detection and quantification – in fact, light elements in general – however, present significant challenges for microscale detection in the scanning electron microscope (SEM). However, recently, a quantitative estimation of the Li content in a LiAlMg alloy [1] was performed successfully using a composition-by-difference (CDM) method based on elemental quantification by EDS and quantitative backscattered electron (qBSE) microscopy and subsequently extended to oxides [2].
In this application note, we show how Cipher can be used to map the structure and elemental composition—including lithium—of hundreds of NMC 811 particles with a sub-micron spatial resolution by Li-CDM.
Methodology
A commercially available NMC 811 powder with nominal lithium composition of 7.3 ± 0.3 wt. % (25.0 ± 1.0 at. %) was analyzed using a Cipher system (model 475.125.70) attached to an FE-SEM. The sample was prepared for analysis by embedding in G2 epoxy and cross-sectioned by broad beam argon milling using a PECS II™ system (model 685.OV); during milling, the sample temperature was maintained below -50 °C to prevent migration of the lithium. The sample was then transferred to the SEM in an inert atmosphere to preserve the native state of the sample using the transfer pod of the PECS II and an iLoadlock™ system.
BSE and EDS analysis were performed using the OnPoint™ (BSE) detector and Octane Elite Super EDS detector of the Cipher system, respectively. The intensity scale of BSE images collected using the OnPoint detector was calibrated for mean atomic numbers 6 – 53 using 5 high-purity standards, Figure 2. All data collection and analysis were performed using DigitalMicrograph® software.


Results and discussion
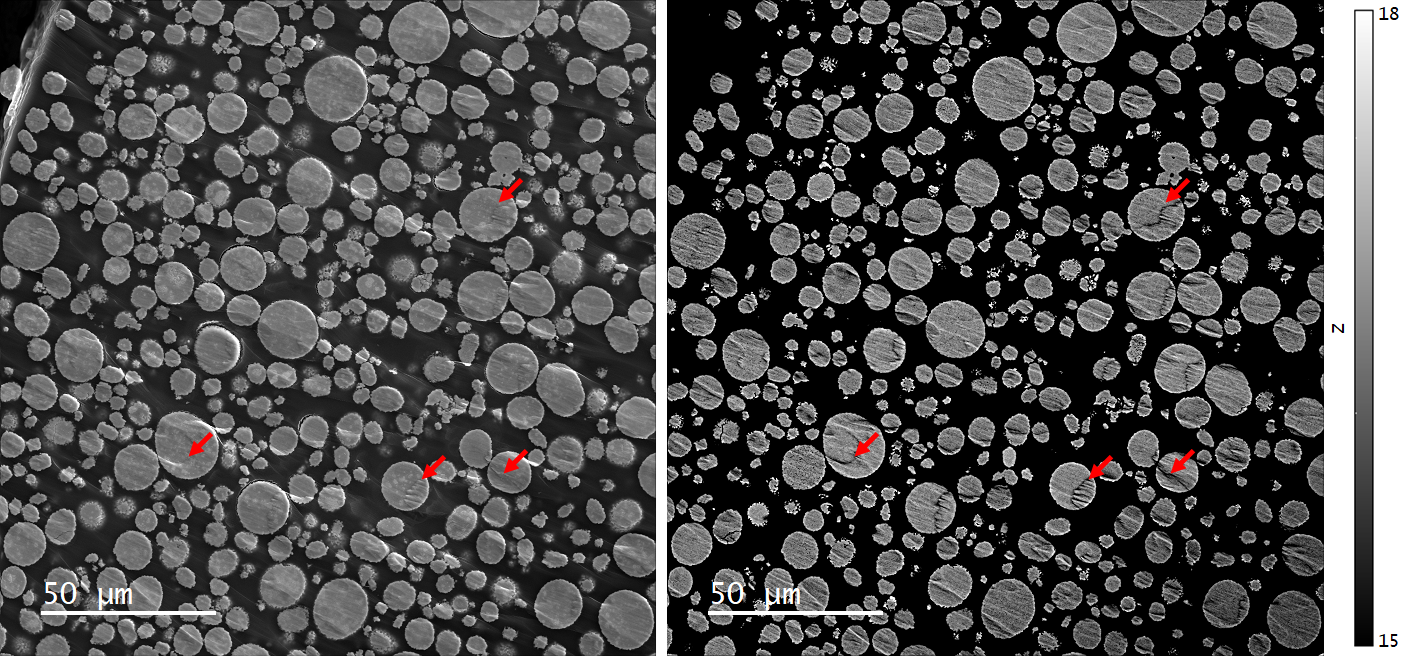
Secondary electron (SE) and qBSE images revealed NMC particles ranging from approximately 5 – 20 µm in diameter. These secondary particles consisted of several hundred smaller primary particles, typically 50 - <1,000 nm in size, Figures 3 and 4.
Comparison of the SE and qBSE images revealed that, in some areas (indicated by red arrows), the qBSE signal included a contribution from topography associated with artifacts in sample preparation in addition to the atomic number contribution used in Li-CDM. Consequently, regions that include significant topography should be disregarded from the compositional analysis by Cipher. Nevertheless, large areas of the sample were found to be suitable.

For quantitative analysis of the BSE signal, an accelerating voltage of 10 kV was selected. At this condition, the effect of channeling contrast—which was significant at lower accelerating voltages (Figure 4a)—was found to be insignificant (Figure 4b), whilst a spatial resolution suitable for analysis of smaller NMC secondary particles was retained.
The distributions of Ni, Mn, and Co in ~500 particles were found by EDS mapping, Figure 5. Quantitative evaluation of the Ni, Mn, and Co content was determined using the standardless eZAF correction method with the background established by physical bremsstrahlung calculation. The mean Ni:Mn:Co ratio was determined to be 8.07:1.0:1.01, consistent with the nominal composition of the NMC 811 powder; however, ~5% of particles were found to have an Mn content ~50% lower than the mean.

The elemental maps and qBSE data were used to calculate the lithium content using Cipher’s composition-by-difference algorithm, Figure 6. Registration of the two data types was performed based on the cross-correlation of the SE images that were captured synchronously with each signal. Corrections for x-y translation, rotation, magnification, and sampling were applied automatically.

The mean lithium content of the NMC powder sample was determined to be 23.8 ± 3.9 at. %, comparing very favorably with the nominal value of 25.0 ± 1.0 at. %. As expected, some regions of the specimen with strong topography produced anomalously high lithium values and should be disregarded (indicated by arrows in Figure 6); however, the intraparticle variation was low, and no significant difference in lithium content was observed between particles.
Summary and conclusions
Cipher has been used to estimate the Li content in ~300 NMC 811 particles, determining a mean lithium content of approximately 23.8 ± 3.9 at. %. This is the first time that the lithium content in cathode materials has been mapped at this length scale, and this exciting result paves the way towards being able to study lithium migration at the microscale during the charge-discharge cycle in battery cells, promising to deliver new insights into structural and compositional evolution over a cell’s lifetime.
References
[1] J.A. Österreicher et al., Scripta Materialia 194 (2021), 113664
[2] J. Lee et al., Microsc. Microanal. 28 (2022), p548-550