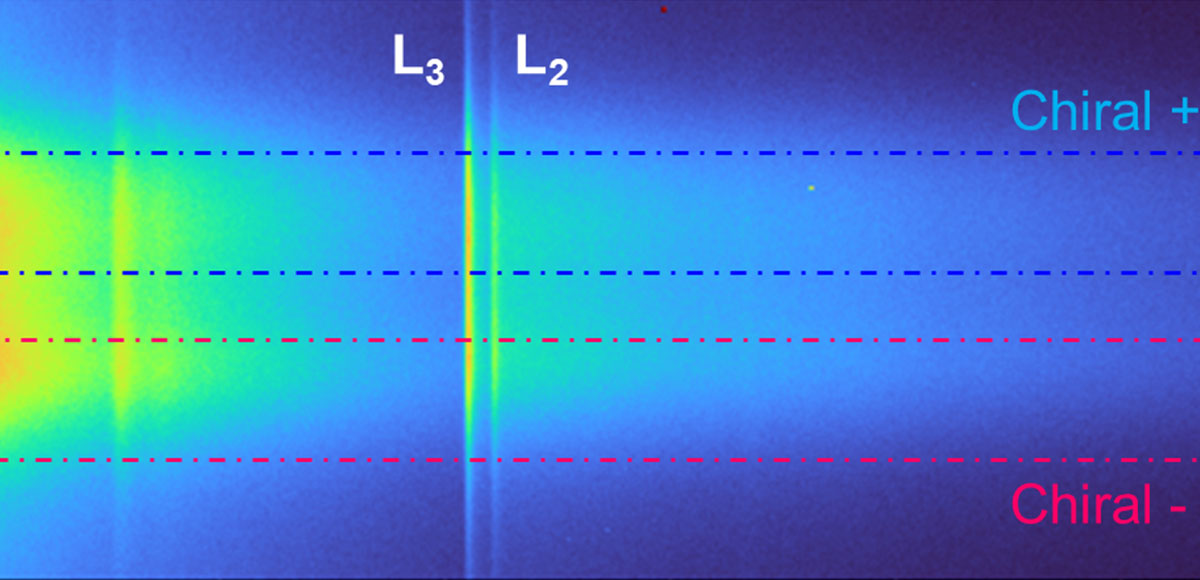
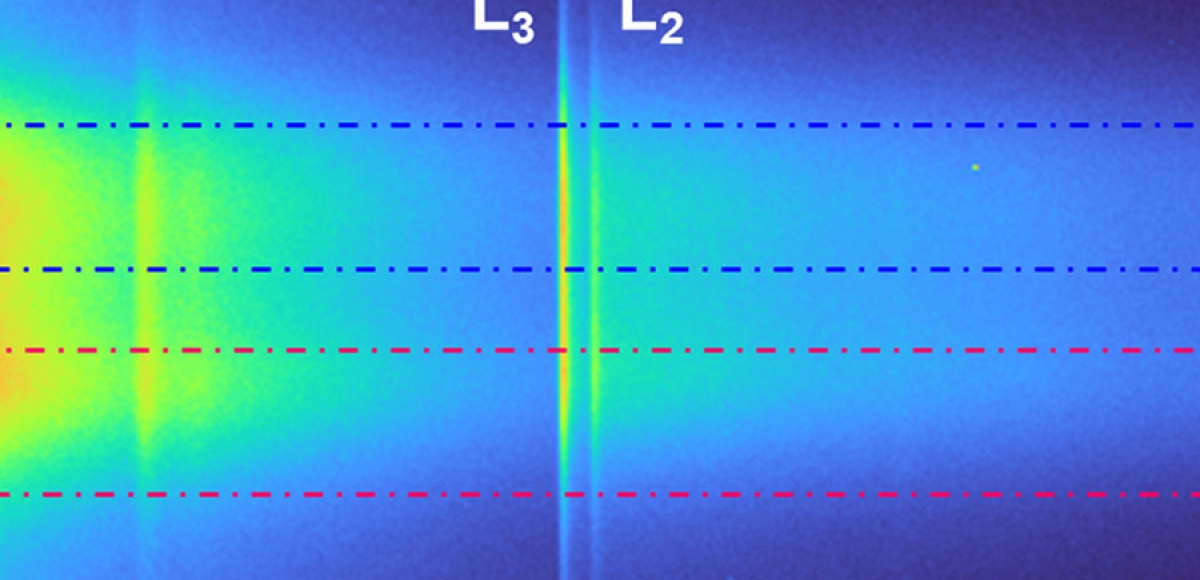
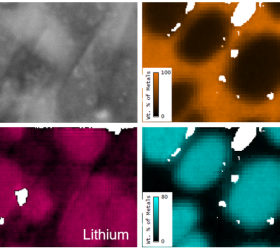
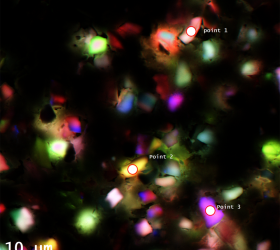
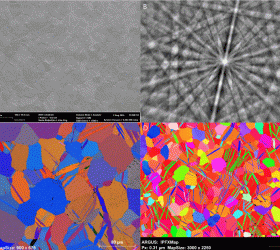
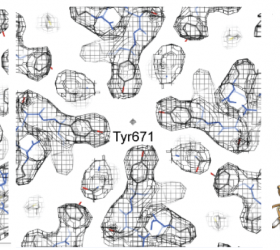
Recent Publications
Phenomenological understanding of the contribution of bulk and grain boundary precipitates on strengthening in prolonged-aged Al-Zn-Mg-Cu aluminium alloys
Materials Today Advances
2025
Strain effects in SrHfO3 films grown by hybrid molecular beam epitaxy
ACS Applied Electronic Materials
2025
NEWS
August 04, 2025
October 29, 2024
EVENTS
Feb
10
2026
Webinar
Germany