Lu, P.; Bai, X.; Ma, D.; Xie, T.; Yan, C.; Sun, L.; Yang, G.; Zhao, Y.; Zhou, R.; Scheres, S. H.; Shi, Y.
First 3D structure of human γ-secretase determined by cryo-EM at 4.5 Å resolution
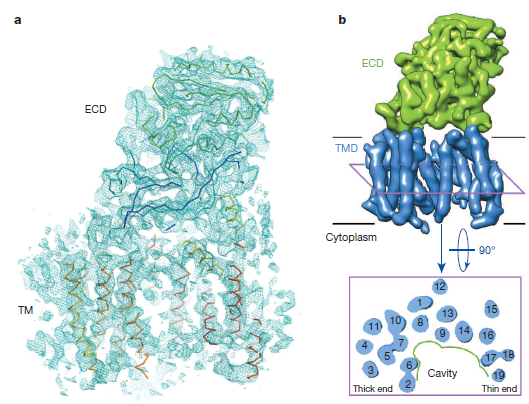
The K2 camera helped determine the three-dimensional (3D) structure of human γ-secretase using cryo-electron microscopy (cryo-EM) single-particle analysis at 4.5 Å resolution in the presence of detergent. This membrane-embedded protease consists of four subunits denoted presenilin 1 (PS1), PEN-2, APH-1 and nicastrin and participates in a number of cellular functions through substrate cleavage. One of the most significant functions of this protease is related to its aberrant cleavage of amyloid-β, which accumulates in the brain and causes Alzheimer’s disease. A key development in determination of this higher resolution structure was the substitution of digitonin with amphipol A8-35, which significantly reduced the disorder resulting from the use of the former detergent. Nature 512, 166 – 170 (2014)