Paolo Longo, Ph.D., Gatan, Inc.
Sample courtesy of Professor Jianfang’s group, Chinese University, Hong Kong
Microscope courtesy of IBM, Fishkill, NY
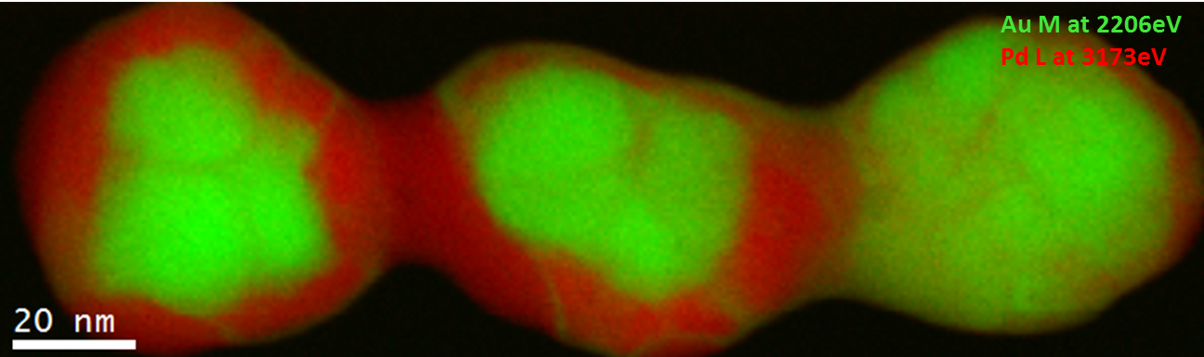
EELS color map of a Pd/Au catalyst particle
Pd/Au alloys have attracted a lot of interest due to their resistance at high temperatures, and this explains their use in several fields, such as CO and hydrocarbon oxidation, synthesis of vinyl acetate monomer, hydrocarbon hydrogenation, and many others. Pd is the catalytic center, whereas the Au has the effect of changing the chemical properties at the surface of the Pd-Au alloy. This change influences the catalytic properties. Hence, the study of the chemistry and the elemental distribution is important to understand the properties of the whole catalyst system. EELS has proved to be a very valuable tool for characterizing such materials.
Methods
DualEELS mode; probe corrected FEI Titan TEM/STEM microscope; X-FEG emission gun; GIF Quantum® ER system
Au M4,5-edges at 2206 eV (green) and Pd L2,3-edges at 3173 eV (red); voltage: 200 kV; data taken in STEM mode; EELS core-loss spectrum (1800 – 3800 eV) exposure time: 49 min; EELS low-loss spectrum (0 – 2000 eV) exposure time: 0.050 ms; beam current: 200 pA