The importance of specimen support film for cryo-EM
An important factor that limits high-resolution cryo-electron microscopy (cryo-EM) data collection is preparing the specimen. An important aspect of specimen preparation is the carbon support film to which the specimen is applied before plunge freezing in liquid ethane. The surface charge, uniformity in thickness, planarity, hole size, and spacing of the carbon support film play a part in successful cryo-EM preparations.
Holey carbon films, used with or without an additional overlay of thin (~ 5 nm) carbon, are commonly used for specimen support for cryo-EM studies of biological macromolecules. These holey support films can be made in the laboratory or be purchased commercially. The preparation methods for most homemade holey supports involve casting a thin layer of resin such as polyvinyl formal (commercially known as Formvar or Vinylec), which is then further treated to produce the perforations. The perforated film is then applied to the surface of an electron microscope grid, and a layer of carbon is evaporated onto the surface to enhance its stability and conductivity when exposed to the electron beam of the TEM. The resin layer is often removed by exposing it to a solvent with the intent of leaving behind just the holey carbon layer. Besides the amount of time and the number of steps required to prepare these support films, it is not easy to get reproducible results with regard to its overall thickness, uniformity, hole size, or hole spacing. Although somewhat expensive, commercially prepared films are an attractive alternative to homemade films because they are sophistically fabricated with a specified thickness, planarity, hole shape, size, and spacing, all with minimal variation from one batch to the next.
Whether homemade or commercially produced, a common aspect of support films is the variability of the surface charge. With some exceptions, such as certain membrane proteins, most biological macromolecules prefer a hydrophilic surface. The visual indication of what occurs as the solution that contains the specimen is applied to the surface of the support film is an important qualitative measure of the surface charge. Ideally, one wants the specimen solution, which is usually anywhere from 2 – 5 μL, to spread out evenly across the surface of the grid such that the majority of the specimen particles partition within the holes of the support without causing any changes to the macromolecular structure of the specimen. If the specimen solution ‘beads up’ when applied to the support film, the amount of specimen that partitions to the holes may be very small, limiting the amount of data one can gather.
Developing a reproducible means for preparing the surface charge of a support film before applying the specimen can improve the quality of the cryo-EM sample preparation and maximize the amount of data gathered from each specimen grid. To render specimen supports hydrophilic, plasma cleaning is now widely accepted as the method of choice. Commercial instruments designed for this purpose consist of a low-vacuum chamber to contain the item to be cleaned and a means to introduce specific mixtures of gases that can be ionized by electrical current to form the plasma. Absorption of electrical energy by the gas increases its temperature causing the ions to increase speed. These energetic ions can then interact with the exposed surfaces of the items being cleaned. Ion bombardment by an inert gas, such as argon, is similar to sandblasting; a small amount of material is removed from the surface. When a gas mixture containing oxygen is used, the bombardment of the surface causes chemical reactions that result in the removal of hydrocarbon contaminants from the surfaces of the items being cleaned. When cleaning hydrocarbon contamination from the surface of fragile holey carbon support films, the plasma must be gentle enough not to break the support during the cleaning process. As a rule of thumb, the specimen supports should be kept in a clean environment and be used within one hour following plasma cleaning.
The Solarus® II is a state-of-the-art, versatile, and easy-to-use plasma cleaner. It provides optimal plasma power for any cleaning condition by incorporating a real-time radio frequency (RF) auto-matching network and a variable RF power supply operating at 0 – 65 W. Mass flow controllers (MFC) for hydrogen, oxygen, and argon gas automatically provide accurate gas flow into the cleaning chamber ensuring stable plasma production during the cleaning process. The unique H2/O2 gas mixture recipe provides superior cleaning with less sputter damage for even the most fragile specimens, and the plasma runs approximately 50% cooler than that from typical gas mixtures such as Ar or Ar/O2. Using the interactive touch-screen interface, every user can obtain consistent results by selecting one of the seven pre-programmed recipes that have been optimized based on the sample type. The spacious side-entry specimen chamber can accommodate up to 50 specimen grids at one time or two TEM specimen holders, the latter of which can be inserted through ports at the front of the chamber. Process cycle rates of less than two minutes ensure high throughput and performance.
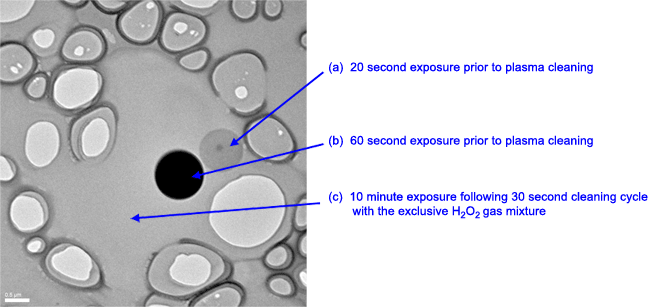

Using a reproducible method for cleaning holey carbon support films is important because it results in a uniform spreading of the specimen solution over the surface of the grid support. With its proprietary H2/O2 gas mixture recipe, the Solarus II plasma cleaner can improve the surface characteristics for holey carbon support films by gently and efficiently removing hydrocarbon contaminants, resulting in consistent and more uniform specimen distribution in cryo-EM preparations and therefore more productive data collection sessions with TEMs.
References
-
Dubochet, J., Groom, M. and Mueller-Neuteboom, S. (1982), Mounting of macromolecules for electron microscopy with particular reference to surface phenomena and treatment of support films by glow discharge.
-
Advances in optical and electron microscopy, Barrer, R. and Cosslett, V. E. (eds.), Academic Press, London, New York. 107-135.
-
Fukami A, Adachi K. (1965) A new method of preparation of a self-perforated micro plastic grid and its application. J Electron Microscopy (Japan). 14(2):112-118.
-
Harris, JR. (1997). Negative Staining and Cryoelectron Microscopy. Oxford: BIOS Scientific Publishers Limited. 12-21.
-
Steinbrecht, RA, Zierold, K. (1987) Cryotechniques in biological electron microscopy. Berlin: Springer-Verlag. 47-54.
The author thanks Dr. David DeRosier and Dr. Daniela Nicastro of Brandeis University, Dr. David Nackashi of Protochips, Inc., Dr. Ken Sautter of Yield Engineering Systems, and Mr. Dick Mitro of Gatan, Inc., for helpful comments.