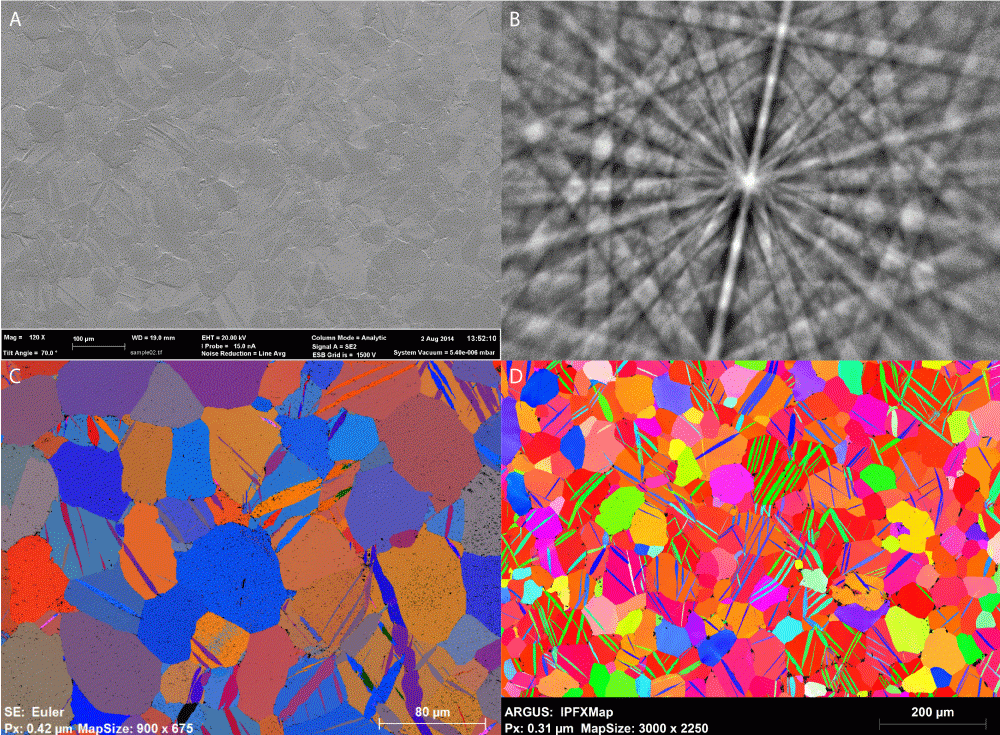
Zircaloy 2
Results courtesy of Department of Materials, University of Oxford. Professor Angus Wilkinson and Dr. Hamidreza Abdolvand. Data acquired on a Zeiss Compact Merlin equipped with a Bruker Quantax EBSD System.
(A) Secondary electron image of the surface of highly stressed Zircaloy 2 alloy showing twin boundaries; (B) BSED Kikuchi pattern of sample; (C) EBSD Euler angle map; (D) IPFZ map, FOV 1 mm x 0.7 mm
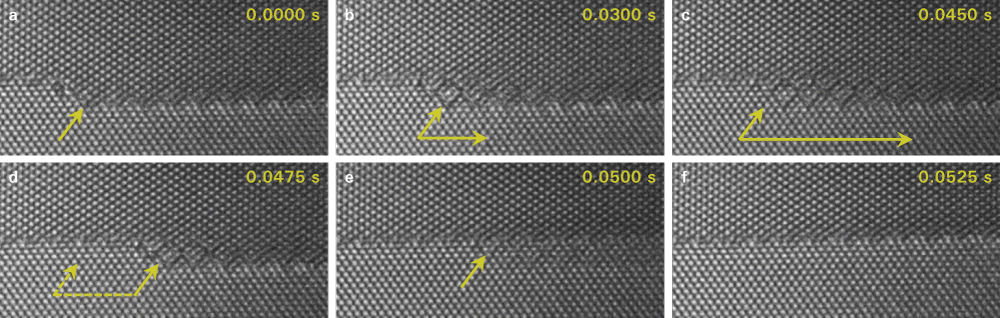
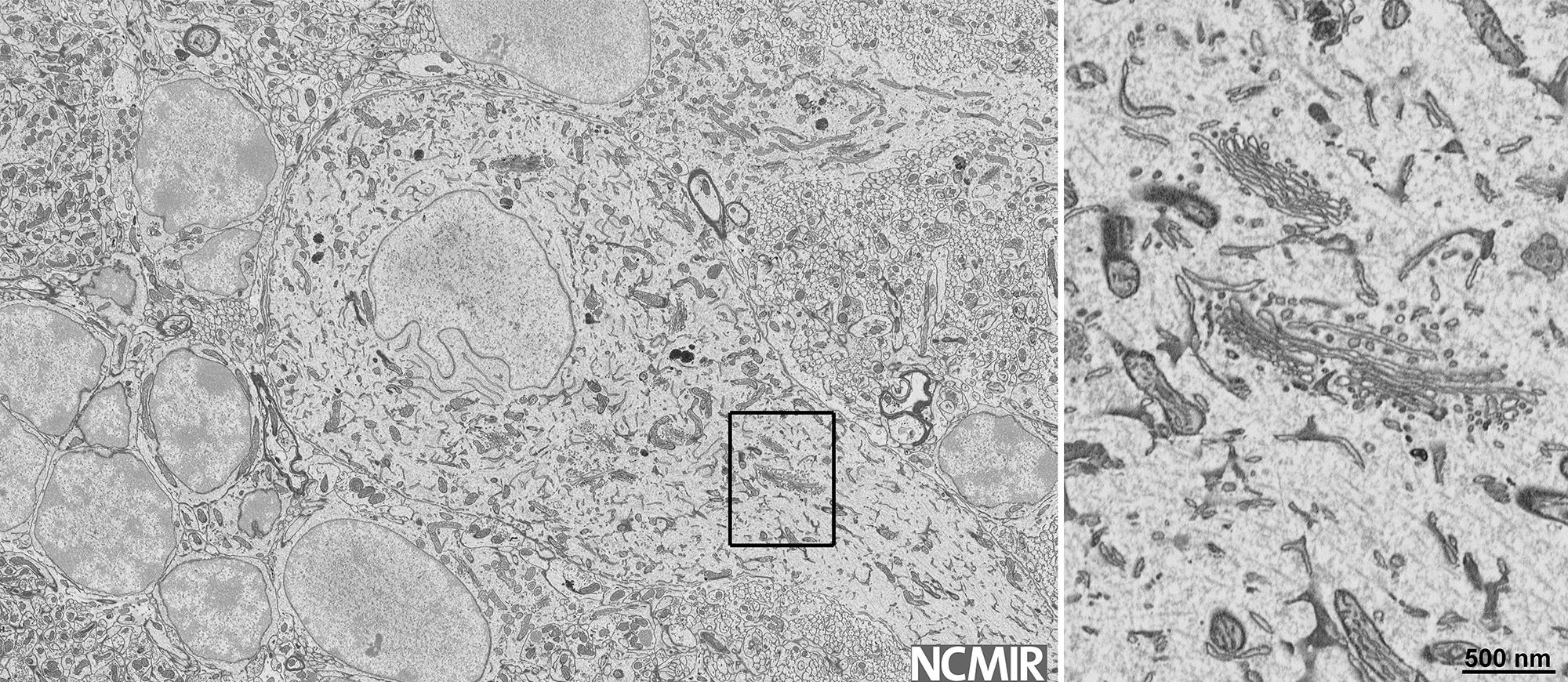
High resolution observations of interface dynamics using a direct electron detection camera
Data courtesy of National Center for Electron Microscopy, LBNL, Berkeley, CA, USA and Faculty of Technology & Metallurgy at U. of Belgrade, Serbia.
4/6 Multilayer step—Last 0.05 s
- Step height n/m=4/6 – 4 planes in lower grain meet 6 planes in the upper grain
- Preserved ABAB…stacking sequence over the step interface
- Buried step parallel to the surface forms during step migration (b-d)
- “Shadow” of the step most likely due to remanence at the surface (e)
This work is supported by DOE/BES/MSD under Contract No.DE-AC02—05CH11231.
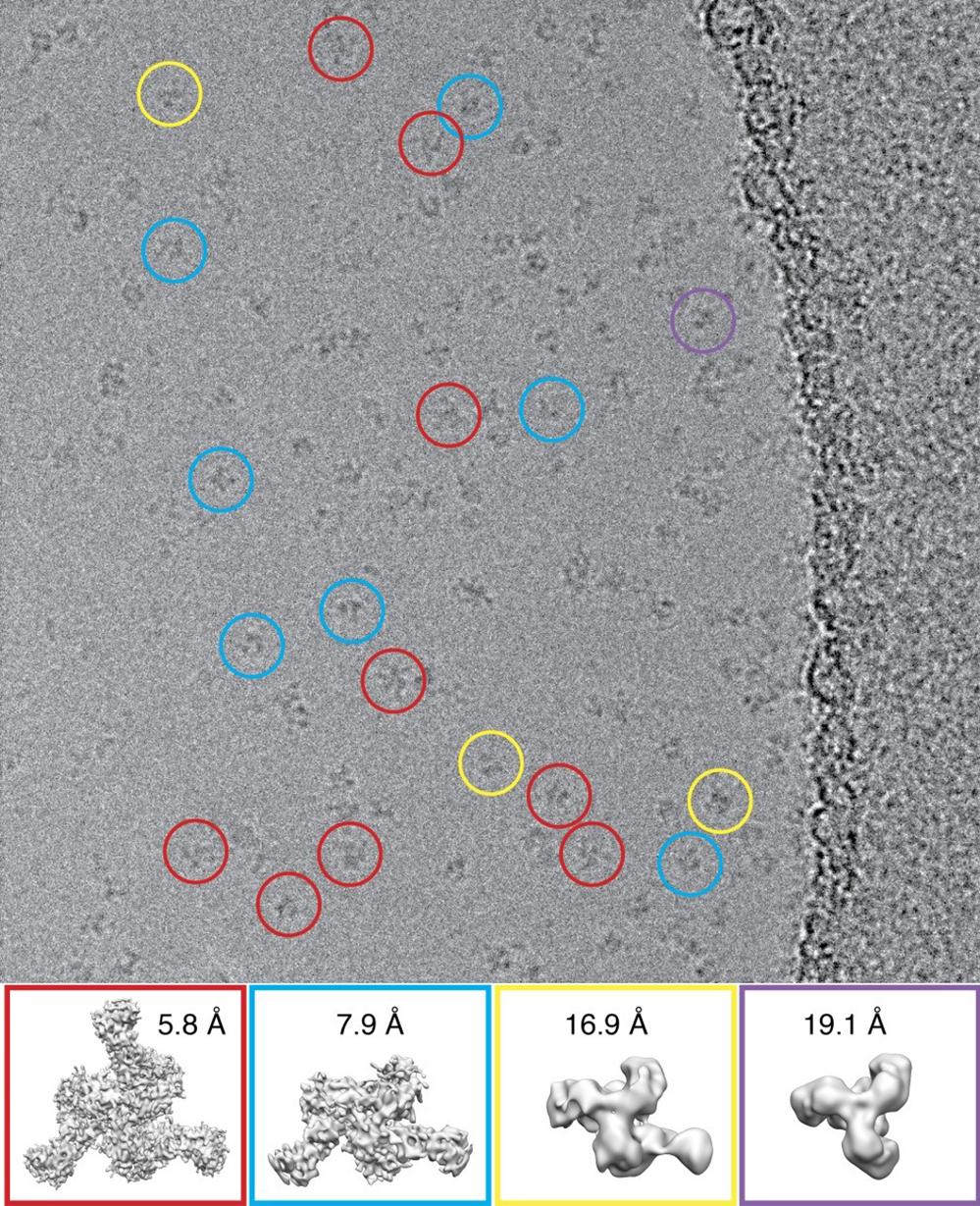
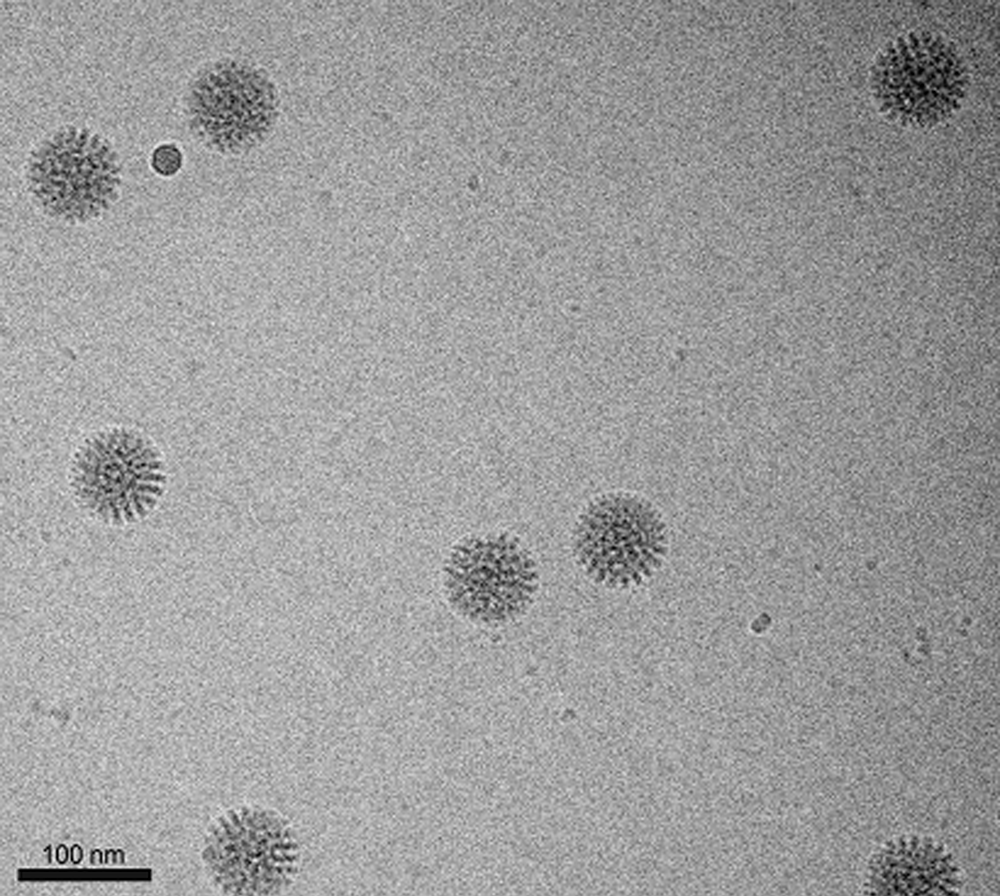
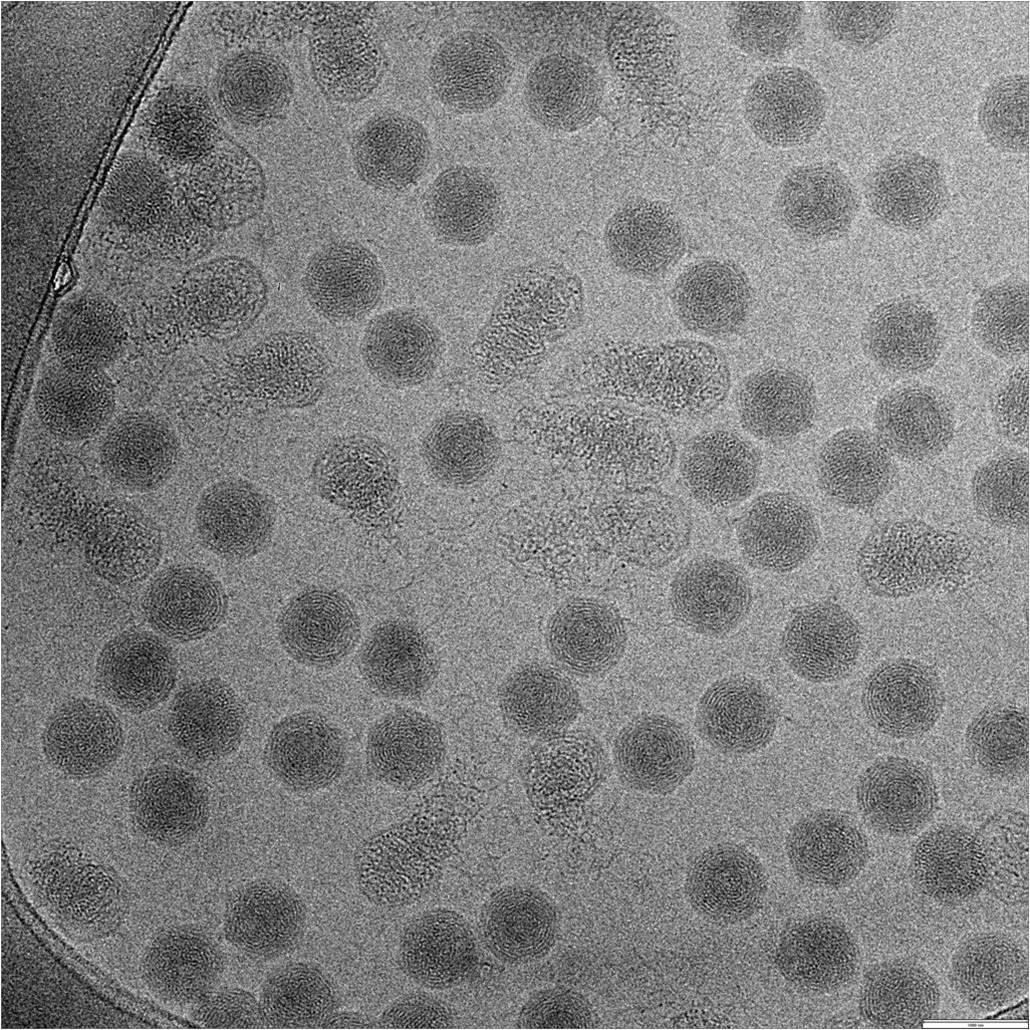
K2 Summit camera enables the study of heterogeneous particles by cryo-EM
High performance of the K2 summit camera gives higher contrast, which is extremely helpful when studying heterogeneous particles. Shown above are 4 different populations of particles, which are found when mixing the HIV-1 envelope glycoprotein trimer with the antigen binding region of an anti-HIV antibody helping to guide rational vaccine development.
Lyumkis et al., 2013 Science
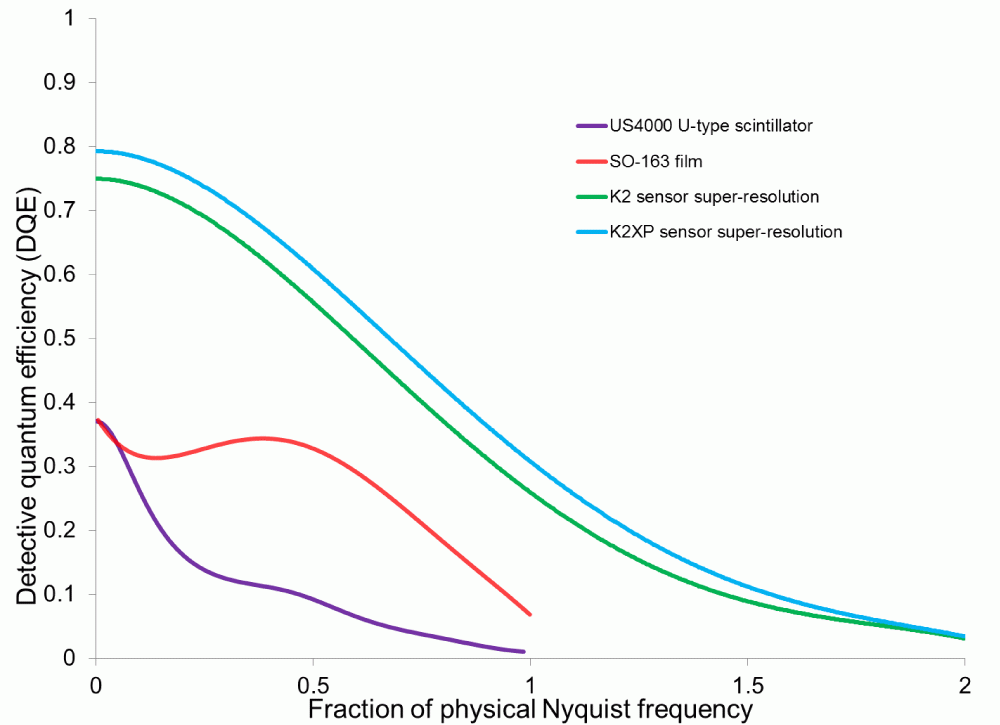
K2XP sensor takes the K2 Summit camera to the next level of performance
Shown above is the DQE curve for the K2® Summit direct detection camera in super-resolution mode using the original K2 sensor and the new K2XP sensor. The K2XP sensor offers a boost in performance of the K2 Summit camera across all spatial frequencies.
Large format image support is critical for throughput
Image courtesy of Mark Ellisman, UCSD/NCMIR
Collect up to 32k x 24k images with the 3View® 2.XP system. Large format image support minimizes the time it takes for the stage to move and settle when tiling large image datasets.
Frozen-hydrated rotavirus double-layered particles
Image courtesy of Dr. Debbie Kelly, Virginia Tech, Carilion
Methods
frozen-hydrated rotavirus double-layered particles (0.01 mg/mL concentration, 2 µL volume)
prepared on Affinity Grids decorated with His-tagged protein A and antibodies against the outer capsid protein, VP6
specimen prepared using Cryoplunge™ 3 instrument with GentleBlot™ technology on C-flat™ 2/1 grids, Protochips, Inc.
specimens examined under low dose conditions at 120 kV using a 626 liquid nitrogen cryo transfer holder
recorded at 50,000x, dose of ~5 electrons/Å2
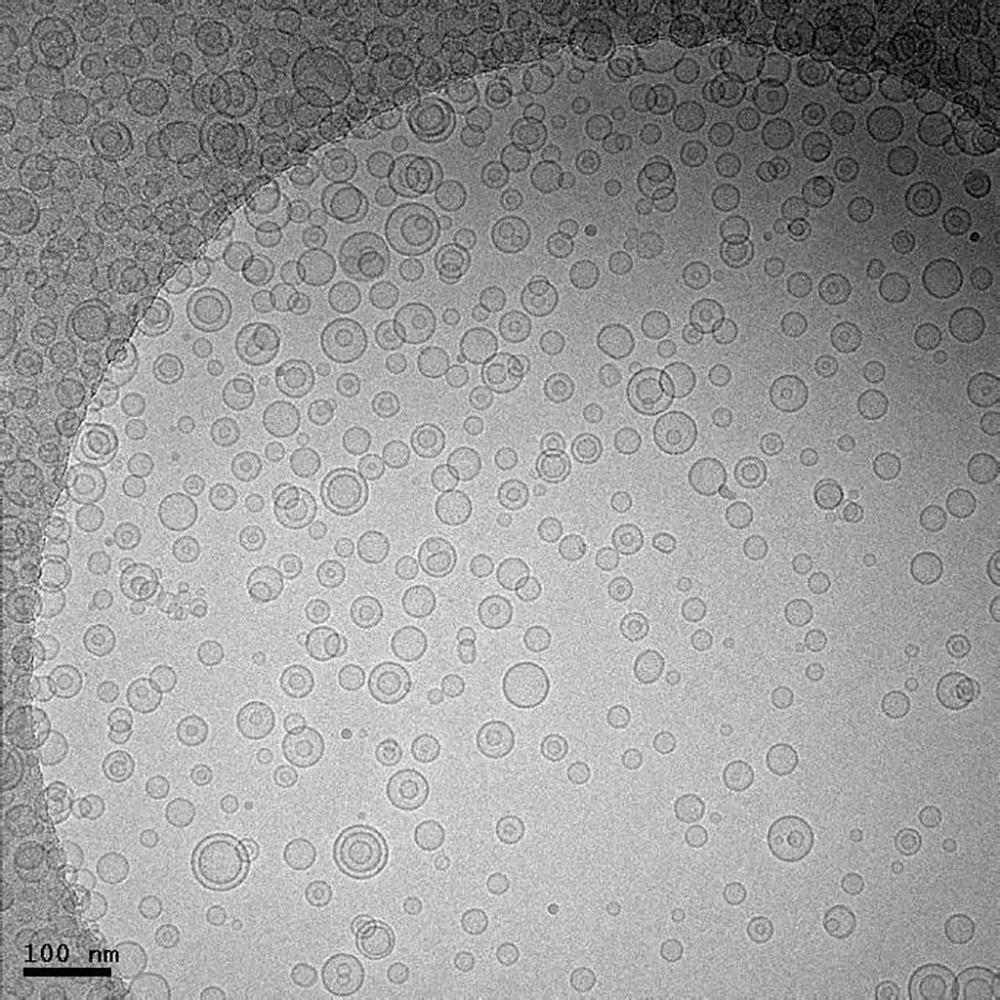
Frozen hydrated lipid vesicles
Image courtesy of Jessica Goodwin and Htet Khant, National Center for Macromolecular Imaging, Baylor College of Medicine, Houston, TX
Methods
prepared using Cryoplunge™ 3 instrument
image was recorded using UltraScan 4000 camera
TEM magnification of 25 kx at 200 keV
electron dose of ~20 e-/Å2
910 multi-specimen single tilt cryo transfer holder
sample prepared on Quantifoil specimen support
used Solarus advanced plasma cleaner prior to freezing
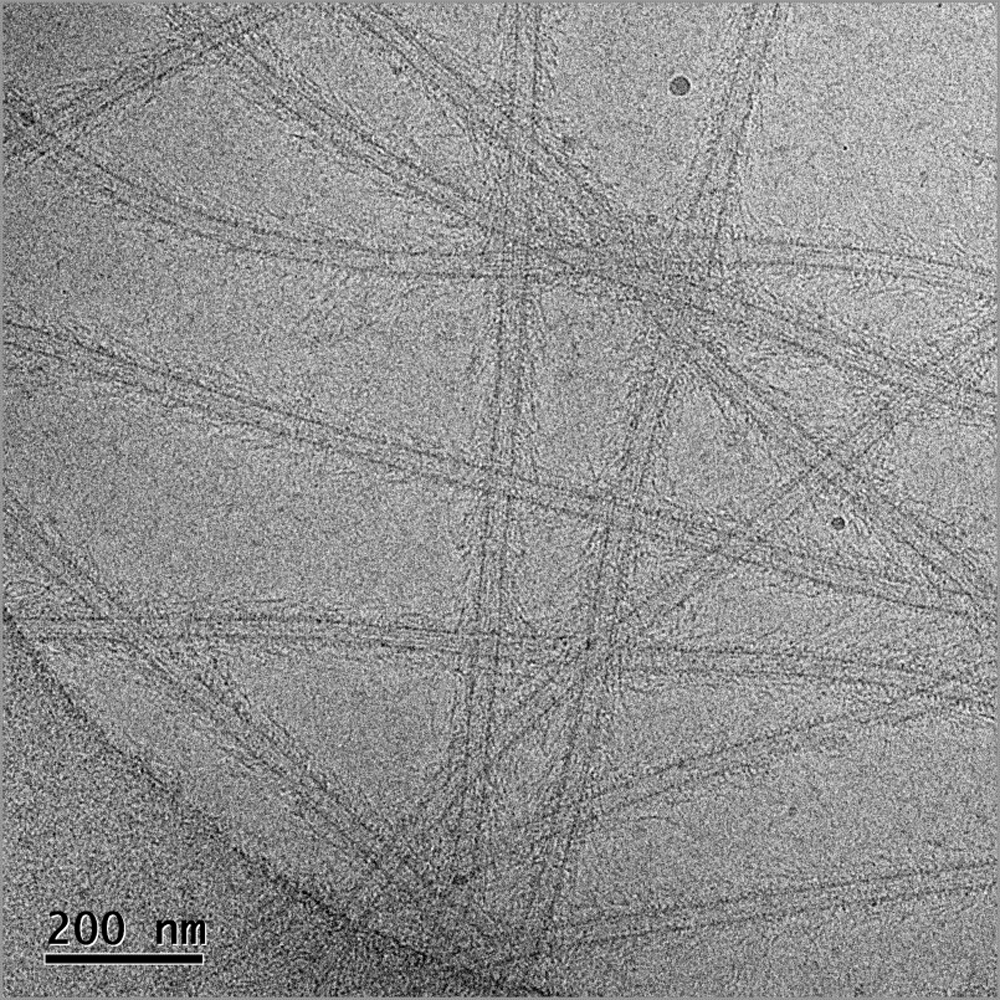
Frozen-hydrated image of the Ndc80 complex decorated microtubules
Image courtesy of Dr. Elizabeth Wilson‐Kubalek, Cell Biology, The Scripps Research Institute, La Jolla, CA
Methods
magnification 30kx
electron dose 40 e‐/Å2
specimens prepared on CFlat™ Protochips, Inc. CF‐2/1‐4C‐50 holey carbon grids
plasma cleaned using the Solarus® advanced plasma cleaning system
frozen hydrated preparations produced using Cryoplunge™ 3 instrument
image recorded at 100 kV on a TEM equipped with a model 626 70° single tilt liquid nitrogen cryo-transfer holder
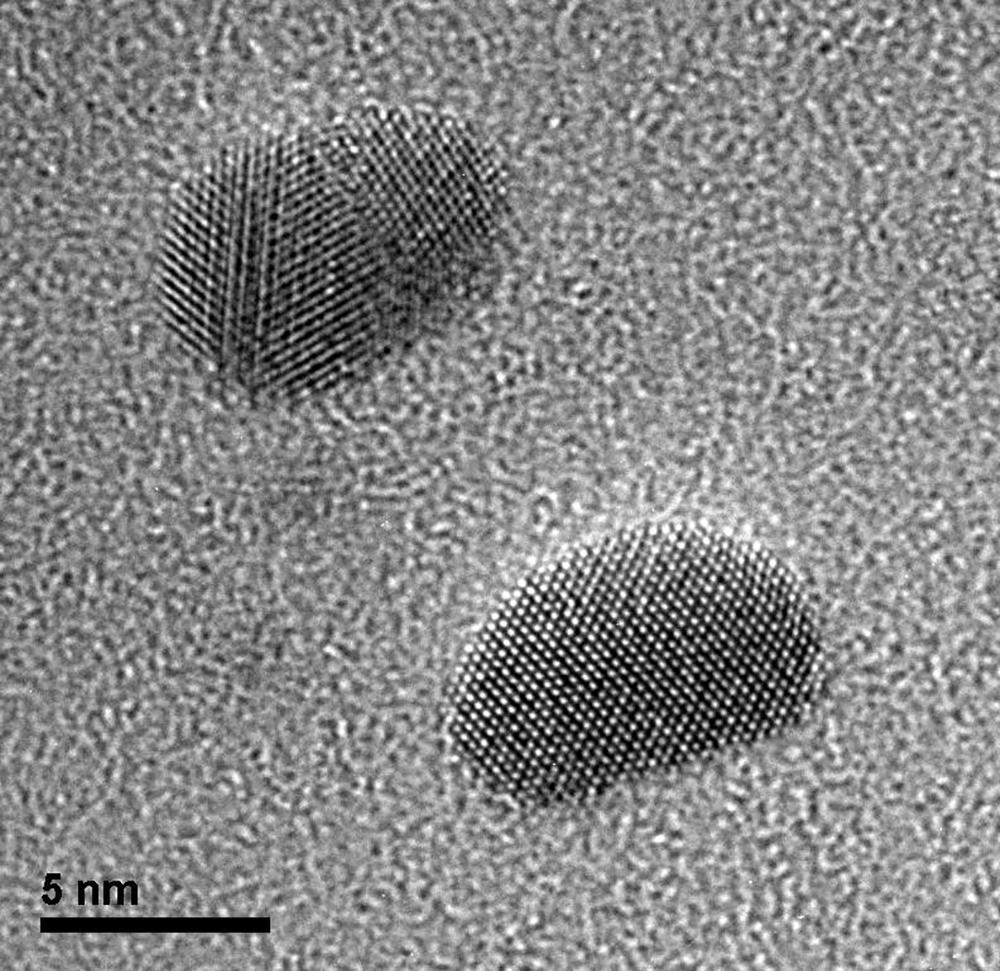
Lattice images of gold particles deposited on a Quantifoil carbon film
Image courtesy of Dr. John Berriman and Dr. Christian Kübel at KIT, Institute of Nanotechnology, Karlsruhe, Germany
Lattice images of gold particles deposited on a Quantifoil® carbon film with grid held at -176 °C using the 914 high tilt liquid nitrogen cryo transfer tomography holder.
Methods
imaged using an UltraScan® 1000 2k x 2k camera
300 kV
8 s exposure
microscope magnification of 380 kx
Cryo-EM images of T7 phage
Image courtesy of Dr. Zheng Liu, Markey Center for Structural Biology, Department of Biological Sciences, Purdue University
Grid was prepared using Cryoplunge™ 3 instrument. Image was recorded at 300 keV using 626 liquid nitrogen cryo-transfer holder.
Scale bar 100 nm
Pages